Chilean degu rodents display behavioral and neuropathological features that resemble human Alzheimer’s Disease
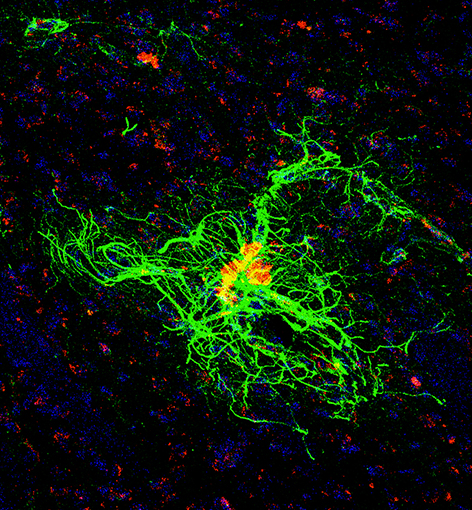
Irvine, CA – December 19, 2022 – Led by researchers from the University of California at Irvine, a new study reveals that a long-lived Chilean rodent, called Octodon degus (degu), is a useful and practical model of natural sporadic Alzheimer’s Disease. The findings were published today in Acta Neuropathologica Communications.
“We found robust neurodegenerative features in cognitively impaired aged degus, including hippocampal neuronal loss, altered parvalbumin and perineuronal net staining in the cortex, and increased c-Fos neuronal activation in the cortex that is consistent with the neural circuit hyperactivity that are commonly reported in human Alzheimer’s Disease patients,” explained corresponding author Xiangmin Xu, PhD, professor and Chancellor’s Fellow of anatomy and neurobiology in the UCI School of Medicine, and director of the Center for Neural Circuit Mapping. “By focusing on a subset of aged degus that show AD-like behavioral deficits and correlative neuropathology, we establish outbred degus as a natural model of sporadic AD and demonstrate the potential importance of wild-type outbred genetic backgrounds for AD pathogenesis.”
This study was motivated by the need to settle earlier debates of whether degus can be a useful natural model of AD. There is a critical need for non-murine, natural animal models for Alzheimer’s research as particularly highlighted by the NIH RFA “New/Unconventional Animal Models of Alzheimer’s Disease.” The handful of published papers on degus of differing genetic backgrounds yield inconsistent findings about sporadic AD-like pathological features, with notably differing results between lab in-bred degus versus outbred degus.
“We suspect that inconsistent findings between different studies may have been due to comparing neuropathology results from laboratory in-bred colonies versus more genetically diverse outbred degus, relatively low statistical power for sample size, and the absence of behavioral screening,” said Xu.
This study revealed that outbred, aged degus possessing both behavioral and neuropathological characteristics that resemble human AD pathologies, have clear advantages over common rodent models (mice and rats) for studying AD. Further, a portion of the outbred degu population naturally develops additional conditions similar to type-2 diabetes, macular degeneration, and atherosclerosis with age, which provides an avenue to investigate AD comorbidities in the degu.
“Our findings, taken together, show spontaneous AD-like correlative phenotypes in cognitive performance and neuropathology in aged, outbred degus. This supports that aged degus are a useful and practical model of natural sporadic AD,” said Xu.
Zhiqun Tan, PhD, an associate researcher with UCI’s CNCM and UCIMIND, and B. Maximiliano Garduño, a graduate student in the UCI Department of Anatomy & Neurobiology, are co-first authors of the paper. Other members of the research team include Todd Holmes, PhD, from the UCI School of Medicine Department of Physiology & Biophysics; Lujia Chen, a graduate student in biomedical engineering at UCI; and their international collaborators Patricia Cogram, PhD, associate professor, and Pedro Fernández Aburto, PhD, from the Institute of Ecology and Biodiversity at the University of Chile. This study was supported by the National Institutes of Health.
Alzheimer disease (AD) is an age-related progressive neurodegenerative disorder characterized by irreversible cognitive decline and specific pathologic lesions in the brain that greatly impair the lives of individuals suffering from the condition. There are approximately 44 million people suffering from AD worldwide, of which over 90 percent of those cases are late-onset and occur sporadically.
https://www.som.uci.edu/news_releases/degu_natural_animal_model.asp


