|
||||||||||||||||||||||

|
||||||||||||||||||||||
Irvine, Calif. – October 15, 2020 – The National Institutes of Health has awarded a team of researchers, led by the University of California, Irvine’s Xiangmin Xu, PhD, a five-year, $3 million grant for a project titled, “Single-Cell Analysis of Aging-Associated 4D Nucleome in the Human Hippocampus.”
Now, as part of the 4D Nucleome consortium, Xu, a professor of anatomy and neurobiology and director of the Center for Neural Circuit Mapping at the UCI School of Medicine, together with MPIs, Carl Wayne Cotman, PhD, a professor of neurology and founding director of the UCI Institute for Brain Aging and Dementia, and Bing Ren, PhD, a professor of Cellular and Molecular Medicine at the University of California, San Diego, will work to build an understanding of aging-associated chromatin architecture changes in single cell nuclei. The three dimensional nuclear organization and its relationship with the regulation of gene expression programs are thought to have widespread and profound implications for human health and disease. The proposed research will help to transform our ability to understand the mechanisms of chromatin organization and function in the context of human brain aging.
“Our proposed research will examine the molecular 4D nucleome changes that occur in human brain region of hippocampus during the course of aging, and the positive effects of physical activity,” said Xu. “With the support of this grant, our team will produce multi-omics cell atlases of the human hippocampus to delineate molecular profiles of normal aging, and uncover potential transcriptional regulatory programs that are impacted by aging. We will share this data with the entire scientific community.”
This cooperative agreement award, number U01DA052769, is funded through the 4D Nucleome (4DN) NIH Common Fund Program, which was launched in 2015 with the goal of developing the tools and resources that would enable the characterization of the three-dimensional structure and dynamics of mammalian genomes and provide deeper mechanistic insights into how the nucleus is functionally organized. For more information visit, www.4dnucleome.org.
About the UCI School of Medicine
Each year, the UCI School of Medicine educates more than 400 medical students, and nearly 150 doctoral and master’s students. More than 700 residents and fellows are trained at UCI Medical Center and affiliated institutions. The School of Medicine offers an MD; a dual MD/PhD medical scientist training program; and PhDs and master’s degrees in anatomy and neurobiology, biomedical sciences, genetic counseling, epidemiology, environmental health sciences, pathology, pharmacology, physiology and biophysics, and translational sciences. Medical students also may pursue an MD/MBA, an MD/master’s in public health, or an MD/master’s degree through one of three mission-based programs: the Health Education to Advance Leaders in Integrative Medicine (HEAL-IM), the Leadership Education to Advance Diversity-African, Black and Caribbean (LEAD-ABC), and the Program in Medical Education for the Latino Community (PRIME-LC). The UCI School of Medicine is accredited by the Liaison Committee on Medical Accreditation and ranks among the top 50 nationwide for research. For more information, visit som.uci.edu.

Irvine, Calif. – September 25, 2020 – In a new study, published in Current Biology, researchers from the University of California, Irvine School of Medicine reveal how subanesthetic ketamine, which is used for pain management and as an antidepressant in humans, is effective in treating adult amblyopia, a brain disorder commonly known as “lazy eye.”
“Our study, demonstrates how a single-dose of subanesthetic ketamine reactivates adult visual cortical plasticity and promotes functional recovery of visual acuity defects resulting from amblyopia,” explained Xiangmin Xu, PhD, a professor of anatomy and neurobiology and director of the Center for Neural Circuit Mapping at the UCI School of Medicine.
Subanesthetic ketamine, commonly used to treat depression and pain, evokes rapid and long-lasting antidepressant effects in human patients. There was evidence that ketamine may control how the nervous system makes structural changes in response to internal and external demands, a process called neural plasticity. But, how the drug worked remained elusive, until now.
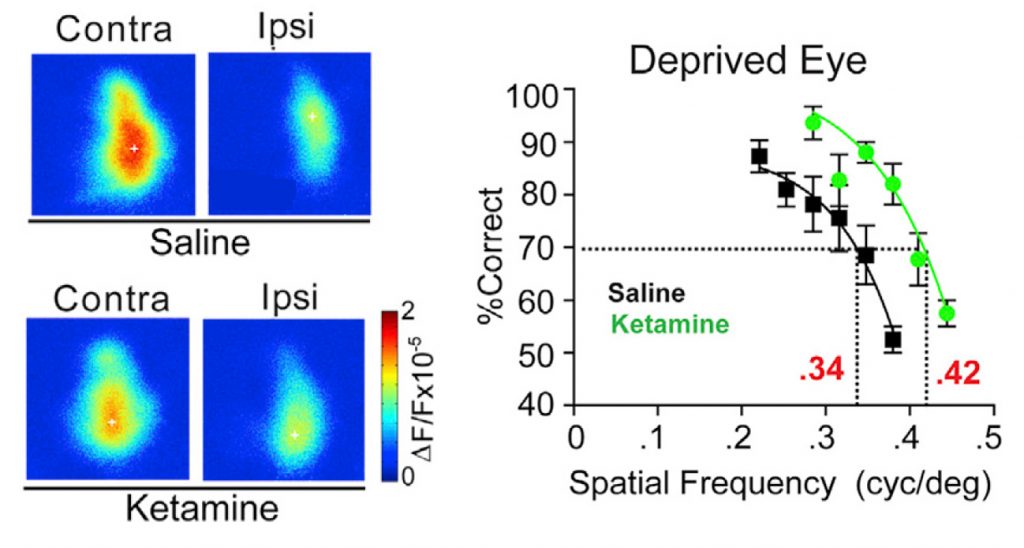
“Our research team showed that ketamine down-regulates NRG1 expression in PV inhibitory cells, resulting in sustained cortical disinhibition to enhance cortical plasticity in adult visual cortex,” said Steven F. Grieco, PhD, a postdoctoral scholar in the Xu lab and lead author. “Through this neural plasticity-based mechanism, ketamine mediated functional recovery from adult amblyopia.” Xin Qiao, PhD, a postdoctoral staff in the Xu lab is a co-first author for the published paper.
Amblyopia is a vision disorder in which the brain fails to process inputs from one eye, favoring the other eye. The condition can result in decreased vision in the affected eye. Each year, between one and five percent of children worldwide, are diagnosed with this condition.
Fast and sustained ketamine actions show promise for therapeutic applications that rely on reactivating adult cortical plasticity. Further testing is needed to determine the full implications of this discovery.
This study was supported in part by the National Institutes of Health grants.

Center for Neural Circuit Mapping established to disseminate molecular tools to the worldwide neuroscience community
Irvine, CA – August 4, 2020 – Researchers from the newly-established Center for Neural Circuit Mapping at the University of California, Irvine School of Medicine evaluate the properties of anterograde and retrograde viral tracers, comparing their strengths and limitations for use in neural circuit mapping. Results were published today as a primer in Neuron.
The article provides a comprehensive comparison of anterograde and retrograde viral and non-viral tracers for neural circuit analysis and describe neural circuit tracing history and background. It also examines the specific viruses used for neuroscience research, and provides essential information to guide other researchers on their choice of viral tracers.
Viral tracers are important tools for neuroanatomical mapping and genetic payload delivery. Genetically modified viruses allow for cell-type specific targeting, and overcome many limitations of non-viral tracers.
“A central goal of modern neuroscience research is to understand the cell-type specific connections between different regions of the brain and the detailed circuit organization within them,” said lead author Xiangmin Xu, PhD, professor of anatomy and neurobiology, and director of the new Center for Neural Circuit Mapping. “Our primer evaluates currently applied anterograde and retrograde viral tracers and provides practical guidance on experimental uses, along with key technical and conceptual considerations for developing new safer and more effective anterograde trans-synaptic viral vectors for neural circuit analysis in multiple species.”
Naturally occurring viruses have been used for neural circuit tracing for decades by exploiting the natural properties of viral propagation and transmission. Genetic modifications of such viruses have led to many improvements for neuroscience applications. In addition to anatomical mapping, genetically modified viral tracers have greatly facilitated functional studies of cell-type specific and circuit-specific neural networks in the brain.
Xu, along with other UCI School of Medicine investigators involved in the primer, including Rozanne Sandri-Goldin, PhD, chancellor’s professor and chair of microbiology and molecular genetics, Todd Holmes, PhD, professor and vice chair of physiology and biophysics, and Bert Semler, PhD, distinguished professor of microbiology and molecular genetics and director for the UCI Center for Virus Research, recently launched the Center for Neural Circuit Mapping (CNCM) at the UCI School of Medicine. The CNCM focuses on neural circuit studies and new viral-genetic technology development. A critical component of the new center is the creation of a viral production facility to disseminate new molecular tools to the worldwide neuroscience community.
“Using new genetic-viral tools, our main goal with the CNCM is to advance the study of neural circuits using animal models to define mechanisms and pathways that underlie neurodevelopmental, neuropsychiatric and neurodegenerative disorders,” said Xu. “Understanding the brain’s neural circuitry is critical for successful translational progress in better treating these diseases.”
The primer article and the new Center for Neural Circuit Mapping are supported in part by the National Institutes of Health’s BRAIN Initiative, the Brain & Behavior Research Foundation and the UCI School of Medicine.
URL: https://som.uci.edu/news_releases/New-guide-for-viral-tracers-neural-circuit-mapping.asp
Irvine, CA – July 21, 2020 – Patients with Alzheimer’s disease frequently suffer from spatial memory loss, such as no recognition of where they are and forgetting where they put their belongings. They often show a wandering symptom, which is also a feature of spatial memory impairment. Until now, the brain network mechanism that causes spatial memory impairment had been unclear.
Published today in Neuron, the study titled “Disrupted place cell remapping and impaired grid cells in a knockin model of Alzheimer’s disease” reveals how the normal brain network function of hippocampus cells, which work to discriminate distinct spatial environments in a process called “remapping,” was disrupted in Alzheimer’s disease. The study, done on Alzheimer’s disease model mice, found that this disruption of hippocampus is most likely caused by activity impairment of the entorhinal cortex, a brain region that supplies information to the hippocampus.
“We recorded the brain cell activity in the hippocampus, which is the memory center of the brain, responsible for spatial memory, among other things,” said Kei Igarashi, PhD, assistant professor in the Department of Anatomy & Neurobiology at the University of California, Irvine School of Medicine and CNCM member. “Our findings could lead to the development of a method to reactivate brain activity of the entorhinal cortex, which may help establish new treatments for preventing the progression of spatial memory impairment in Alzheimer’s disease patients.”
Igarashi has been studying brain network mechanisms for Alzheimer’s disease since he started his lab in 2016. “Our memory comes from activities of the brain network. To find out the cure for memory impairment in Alzheimer’s disease, we need to understand how the network function is impaired,” he said.
Original URL: https://www.som.uci.edu/news_releases/brain-network-mechanism-Alzheimers.asp
Epigenomic analysis could lead to early detection and new therapies
Irvine, CA – May 12, 2020 – A team of researchers from the University of California, Irvine and San Diego have been awarded $3.8 million by the National Institute on Aging to conduct an epigenomic analysis of neural circuits in the brain. By revealing molecular changes that occur during the course of Alzheimer’s disease (AD), the team hopes to identify new therapeutic targets and molecular biomarkers for early detection and better treatment.
The interdisciplinary research team is led by multiple principal investigators, including Xiangmin Xu, PhD, a professor of anatomy and neurobiology and director of the Center for Neural Circuit Mapping at the UCI School of Medicine, Carl Cotman, PhD, a professor of neurology and founding director of the Institute for Brain Aging and Dementia at the UCI School of Medicine, and Bing Ren, PhD, a professor of cellular and molecular medicine and director of the Center for Epigenomics at the UCSD School of Medicine.
The team will study how the epigenome of key cell types in neural circuits shapes hippocampal circuit activity and behaviors during AD progression. The proposed research, conducted using mouse models that mimic the neurodegenerative disease, will involve the use of single cell genomic technologies coupled with functional circuit mapping and behavioral analysis.
“Our goal is to reveal the molecular changes that occur during the course of the disease, that impact learning and memory, and identify a path toward early detection and new drug therapies for Alzheimer’s disease,” said Xu.
Alzheimer’s disease is the most common cause of progressive dementia (memory and cognitive loss) in older adults and a growing major health concern in the U.S. Currently, more than 5.5 million Americans may have dementia caused by AD.
“Currently, there is no cure for Alzheimer’s disease,” said Xu. “And, as millions of people are affected by this debilitating condition, it is increasingly critical that we develop better early diagnostic tools and new treatment strategies to care for them.”
URL: https://www.som.uci.edu/news_releases/Xu-awarded-NIA-grant-to-study-Alheimers.asp
We are pleased to announce a new research Center for Neural Circuit Mapping. Leveraging their multiple NIH BRAIN Initiative Awards, other NIH grants and collaborations, SOM faculty Xiangmin Xu, Rozanne Sandri-Goldin, Todd Holmes and Bert Semler developed the Center plan which was recommended by the Dean’s Research Council and approved by the Dean on January 27, 2020.